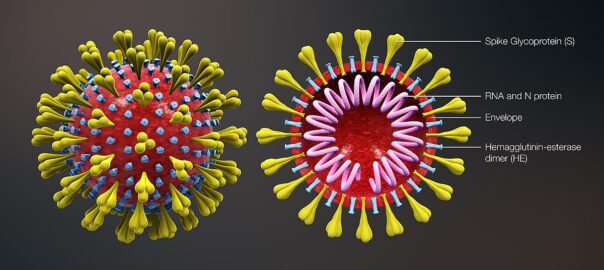
[Above graphic is a combined image from a 3D medical animation, depicting the shape of the coronavirus as well as the cross-sectional view. Image shows the major elements including the Spike S protein, HE protein, viral envelope, and helical RNA (Image by https://www.scientificanimations.com; used under the Creative Commons Attribution-Share Alike 4.0 International license.]
By Kent R. Kroeger (Source: NuQum.com, September 22, 2020)
In May, the University of Minnesota’s Center for Infectious Disease Research and Policy (CIDRAP) — one of the world’s leading research centers on infectious diseases — issued a warning about any expectations of a coronavirus vaccine being available soon or 100 percent effective once available.
Among CIDRAP’s recommendations for policymakers were these two warnings:
States, territories, and tribal health authorities should plan for the worst-case scenario, including no vaccine availability or herd immunity.
Risk communication messaging from government officials should incorporate the concept that this pandemic will not be over soon and that people need to be prepared for possible periodic resurgences of disease over the next 2 years.
Five months later, their cautious words remain relevant.
While the world may be closer than ever to its first regulatory-approved coronavirus vaccine — at least nine vaccines are already in Stage 3 testing — there is a concern among scientists that this first vaccine may not be effective enough to achieve herd immunity (estimated to be around 60 to 70 percent of a population) and could discourage the development of significantly better alternatives.
This month, China announced it has started to deploy two state-approved coronavirus vaccines— both developed by Sinopharm, a state-owned pharmaceutical company — and has already vaccinated over 100,000 people.
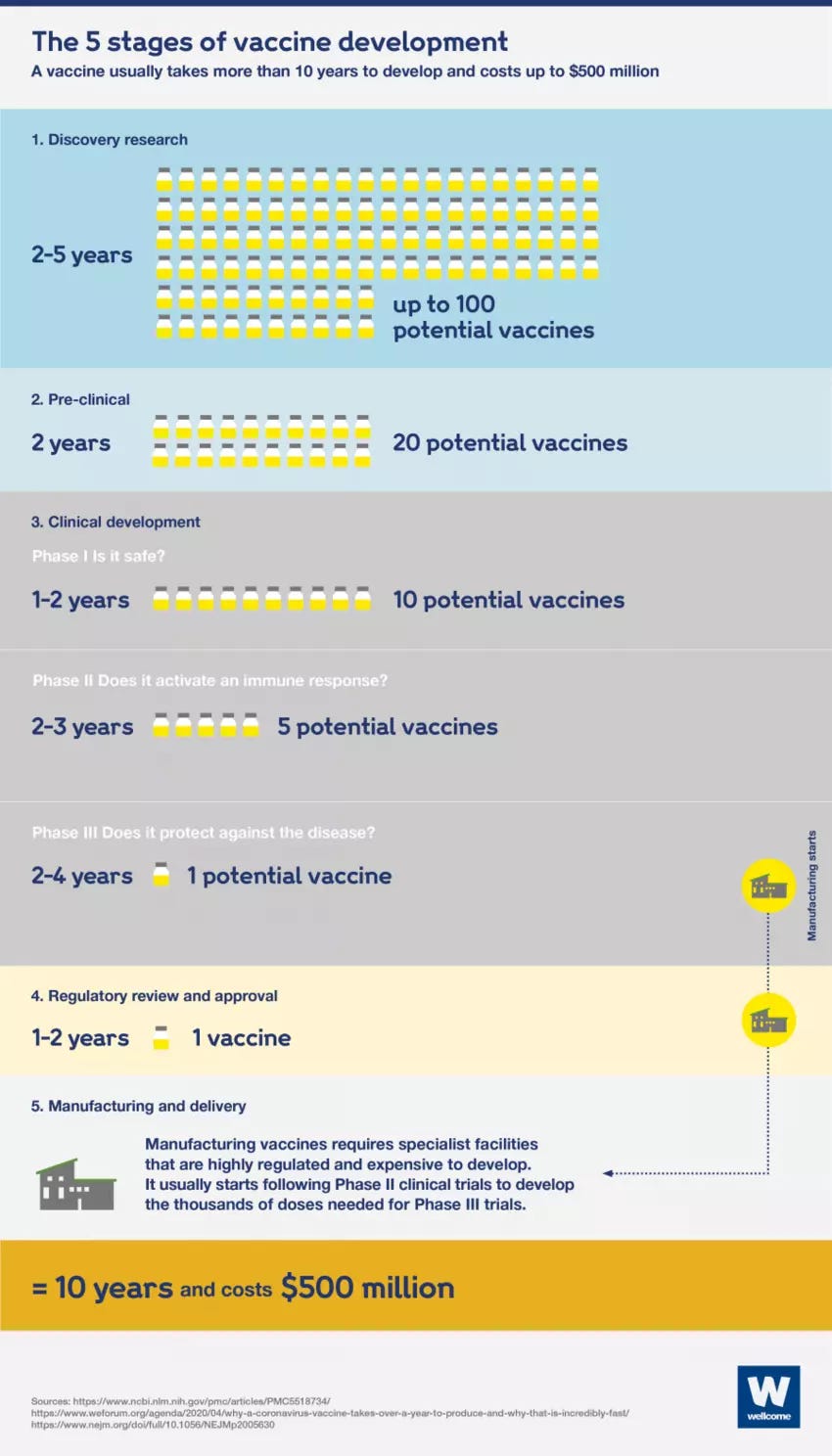
Remarkable is that China is doing this while still in Phase 3 trials for the vaccines (see Figure 1 below for a description of the five stages/phases in vaccine development).
In addition to China, Russia has also approved a new coronavirus vaccine.
Scientists outside of China are predictably concerned and skeptical of China’s aggressive vaccine rollout.
“One needs to carefully conduct clinical trials of adequate size with adequate time for follow-up, look at both efficacy and safety, and those data have to be very carefully reviewed before you start giving the vaccine to people outside of a carefully designed clinical trial,” Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins, told Vox’s Lili Pike.
Phase 3 trials are critical as they involve around 30,000 test subjects and are designed to reveal rare, adverse reactions to test vaccines. For example, if just 1 out of 30,000 vaccine recipients (0.33 percent) has a fatal reaction to an otherwise highly-effective vaccine (say, 90%), that could translate into 260,000 vaccine-related deaths if the vaccine were given to the entire world population.
Figure 1: The 5 Stages of Vaccine Development
However, John Moore, an immunologist at Weill Cornell Medical College, believes China, given its current low infection rates, could afford to wait until Phase 3 trials are completed in order ensure a safe and effective vaccine.
But Moore’s calculus ignores a more powerful dynamic behind China (and Russia) aggressively rolling out coronavirus vaccines far ahead of standard practice in new vaccine development, which typically takes around 10 years.
The fastest development ever was for the mumps vaccine which took four years from start to final regulatory approval.
The economies of China and Russia have been deeply hurt by the coronavirus pandemic (as have all world economies) and there is a strong incentive to end this pandemic as soon as possible — even at the risk of exposing their own citizens to potentially unsafe or ineffective vaccines. The cost-benefit analysis in autocratic societies is fundamentally different than in capitalist democracies such as in the U.S. and European countries.
If China and/or Russia are successful with their early vaccine deployments, they will become the model example for future Lean Six Sigma workshops.
Somehow China and Russia have done in seven months what typically take seven years.
A Half-Baked Cost-Benefit Analysis
The following cost-benefit analysis is meant merely as a thought experiment and is not a formal exercise in risk management. However, it is intended to loosely approximate the analyses underlying the decision by the Chinese and Russians governments to accelerate their vaccine developments.
In the following analysis of an hypothetical early rollout vaccine, these assumptions were used:
- The coronavirus infection fatality ratio equals 0.0084, the most recent CDC estimate (i.e., 0.84 percent of those who contract the virus will die).
- All world citizens (7.8 billion) are vaccinated by the early rollout vaccine and at roughly the same time.
- The early rollout vaccine has a fatality ratio of 0.0033 (i.e., 0.33 percent) — an extremely high ratio that would never be approved by a U.S. or European regulatory body.
- Calculations of total coronavirus deaths (coronavirus deaths + vaccine deaths) are based on a vaccine effectiveness rates of 40%, 60%, 80%, and 90%. (Note: Most vaccines are between 85 and 95 percent effective, according to OurWorldInData.org)
- A coronavirus-related estimate of worldwide deaths assumes everyone is either effectively vaccinated or ineffectively vaccinated. Among those ineffectively vaccinated, they will either die from the vaccination or contract the virus. Those with highly-adverse reactions to the vaccine are rolled into the vaccine fatality rate.
- If no vaccine is ever developed, everyone contracts the virus at roughly the same point in time.
- This analysis ignores retransmission.
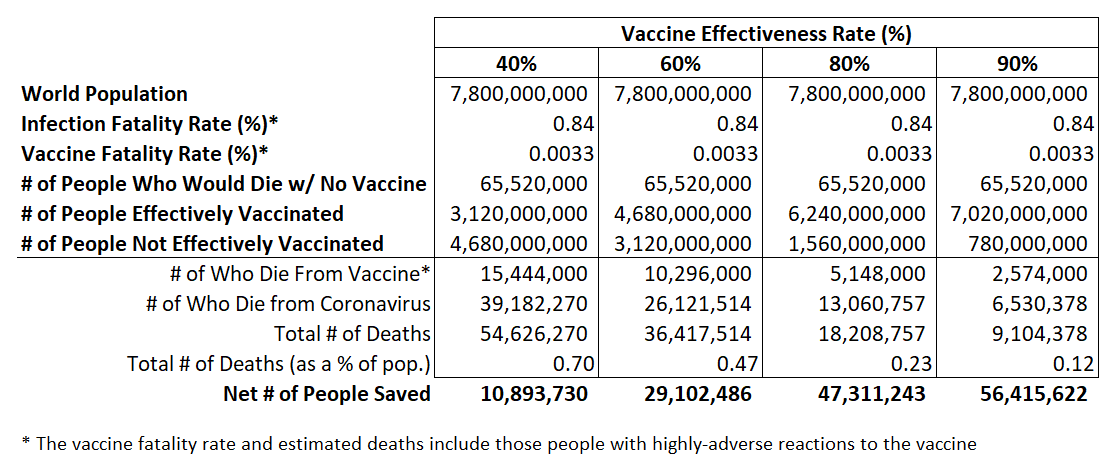
Figure 2 shows the estimated total number of coronavirus-related worldwide deaths for each vaccine effectiveness rate. It is important to note that this is a near worst-case scenario in terms of the vaccine fatality rate (VFR). A vaccine with a VFR of 0.0033 would never be approved by regulators. In the real world, due to strict development requirements, vaccines are extremely safe and this hypothetical cost-benefit analysis is not meant to challenge that scientifically-supported fact.
Figure 2: Hypothetical Cost-Benefit Analysis of Early Rollout Vaccine
Nonetheless, an analysis like the one in Figure 2 is being done across the dozens of pharmaceutical and research institutes working on a coronavirus vaccine right now.
Given that many epidemiologists are estimating that the herd immunity rate for the coronavirus is probably between 60 and 70 percent, a vaccine effectiveness rate less than that might not halt the epidemic.
But as Figure 2 shows, in a near worst-case scenario — a 60 percent effective vaccine with a high fatality rate — such a vaccine could save a net of 29 million people (i.e., the number of people who would have died without a vaccine [65.5 million] minus those who would die with a full vaccine rollout [36.4 million]).
Would you approve of a vaccine with that outcome? I wouldn’t. The Federal Drug Administration most certainly wouldn’t. But would China or Russia? Maybe.
The U.S. gross domestic product (GDP) shrank 9.5 percent in 2020 Quarter 2 due to the coronavirus. In the same period, the Organisation for Economic Co-operation and Development (OECD) area saw their economies fall by 9.8 percent.
As for China, its GDP fell 6.8 percent in 2020 Quarter 1 due to the coronavirus (though it did rise by 3.2 percent in 2020 Quarter 2, according to the Chinese government).
In turn, Russia has watched the price of oil — one of its most important exports — fall from $54-a-barrel in late-September 2019 (WTI crude) to $39-a-barrel (as of 22 Sep 2020). Likewise, natural gas prices have fallen from $2.43 (USD/MMBtu) in late-September 2019 (NYMEX natural gas futures) to $1.79 (USD/MMBtu) as of 22 Sep 2020.
Undeniably, China and Russia both depend heavily on a strong world economy, including economic activity with the U.S. and the OECD countries. The quicker the end to this pandemic, the sooner their economies can fully rebound.
And what about the billions of people in the developing world who are going to be among the last to get a coronavirus vaccine given that a small number of wealthy countries — representing just 13 percent of the world’s population — have already purchased 51 percent of the world’s coronavirus vaccine supply before its been approved and mass-produced?
If China and Russia have produced reasonably effective and safe coronavirus vaccines, they could become heroes to the developing world, who currently face the likely prospect of multinational pharmaceutical companies — that have already extracted billions of dollars from their governments to encourage production an effective coronavirus vaccine — extracting even more billions in profits once they produce an approved vaccine.
Still, a 60 percent effective vaccine under our simplifying assumptions here would still result in 36 million coronavirus-related deaths. That’s a lot. In nine months, the coronavirus has killed around 1 million people worldwide.
By rough comparison, AIDS/HIV has killed 33 million people worldwide since its identification in 1981 (or about 850,000 per year).
History has proven relatively wealthy people can tolerate large numbers of premature deaths as long as its not them. The real question is, will they tolerate China or Russia threatening profits of U.S and European pharmaceutical companies?
Vaccine Failures in the Past
In history, two vaccines are often cited as examples of how things can go wrong with vaccines rolled out too early or carelessly.
The “Cutter Incident” in 1955 resulted in 10 deaths and 164 cases of permanent paralysis after 200,000 people received a polio vaccine that had been improperly produced. That’s an adverse reaction rate of 0.09 percent. The “Cutter Incident” was 26 times more lethal than the 0.0033 percent vaccine fatality rate assumption made in this analysis.
In 1976, another vaccine debacle occurred in the U.S. where within 10 weeks approximately 45 million people were vaccinated for the “swine flu.” The vaccinations stopped however after few cases of the virus ever developed and around 450 Guillain-Barré syndrome cases emerged, resulting in 53 deaths (i.e., 0.001 percent of swine flu vaccine recipients had a highly-adverse outcome).
A personal anecdote:
My family received the swine flu vaccine in 1976 resulting in my father experiencing a severe allergic reaction to it. As told to him by his doctor, since my father had an egg allergy (though minor), he may have reacted to the swine flu vaccine because it had been grown in eggs. However, my father received many flu vaccines after that (and, in all likelihood, having been grown in eggs) and never had a similarly severe reaction.
A few afterthoughts
I want to emphasize this essay is not a tirade against vaccines or the value a strict regulatory standards in their development.
There is no substitute for good science.
But it appears the apparently premature rollout of a coronavirus vaccine in China will end in one of two outcomes: (1) an epic failure that will go down in history as how not to develop a vaccine during a global pandemic, (2) or the start of a revolution in how such vaccines will be developed and approved going forward.
Is it possible regulatory authorities in wealthy countries are too risk averse in applying laws, standards and rules regarding vaccine approvals? Given the many advancements in bioscience over the past two decades, can safe and effective vaccines in some cases be turned around from start to finish in under a year?
We may find out one way or another very soon.
- K.R.K.
Comments can be sent to: nuqum@protonmail.com
or DM on Twitter at: @KRobertKroeger1